TL;DR Summary
Executive Summary
-
Industry
-
Dental Products Manufacturing (Medical Device OEM)
-
Company Profile
-
$300M revenue, 1,500 employees, FDA-regulated environment
-
Challenge
-
FDA date-stamp verification requiring costly dual signatures at every changeover
-
Solution Tested
-
AI-assisted vision system with neural network and
JSONintegration over OPC-UA to MES -
Investment
-
$25,000 POC budget over 3 months
-
Outcome
-
Strategic failure that validated human-centered approach
-
Alternative Solution
-
Andon cord system reducing changeover verification time
-
Key Learning
-
Sometimes people remain the optimal solution - fail fast, scale what works
The Manufacturing Challenge
Regulatory Compliance Reality
A leading dental products manufacturer faced a costly compliance bottleneck that was consuming 2 hours of production time daily. FDA regulations required visible and indelible date stamps on primary containers - specifically, custom 5-digit expiry dates capturing the chemistry lifecycle inside each syringe.
Baseline Process Metrics
- 4 changeovers per day requiring dual verification
- 30 minutes average changeover time per event
- 2 hours daily spent on signature acquisition
- 100% manual process - operator plus supervisor wet ink signatures
- Zero automation - paper batch records only
The company's continuous improvement culture drove exploration of electronic verification systems that could potentially eliminate the dual-signature requirement while maintaining or exceeding human accuracy levels.
The Innovation Opportunity
Traditional quality management systems in FDA-regulated environments rely heavily on human verification. However, the repetitive nature of date-stamp verification presented an apparent opportunity for computer vision automation.
"We needed to prove whether AI could reliably distinguish between similar digits under magnification while meeting FDA traceability requirements," explains the project stakeholder.
The AI Vision Solution Architecture
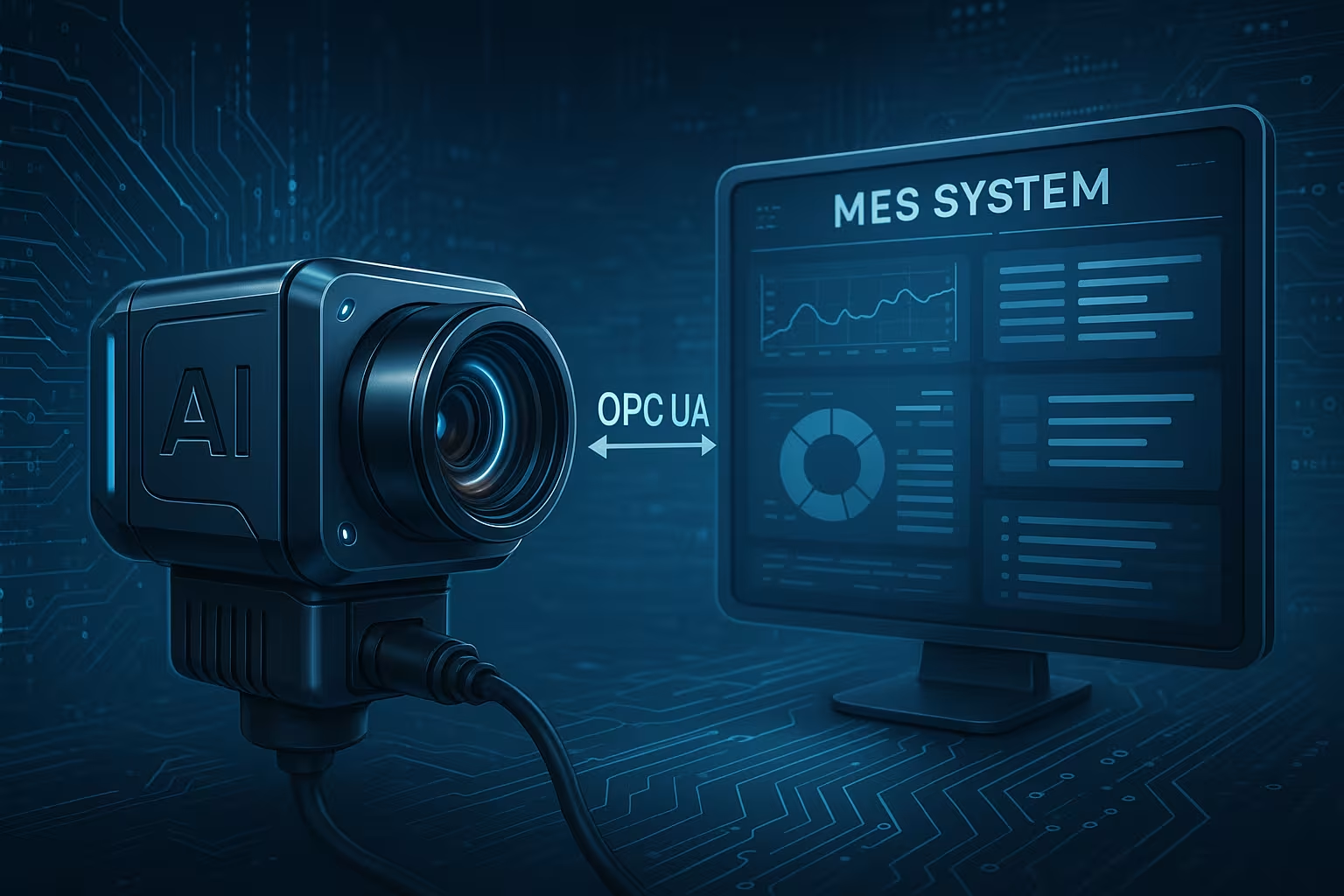
Technical Implementation Design
The proof-of-concept centered on a specialized vision system designed to integrate seamlessly with existing manufacturing execution systems (MES):
- Specialized lightbox with controlled lighting conditions
- Local camera system for high-resolution image capture
- Neural network processing trained on production samples
- JSON integration over OPC-UA with MES for date code verification
- Pass/fail feedback loop to control manufacturing workflow
Integration Architecture
|
Component |
Specification |
Purpose |
|---|---|---|
|
Camera System |
High-resolution with specialized lightbox |
Controlled image capture environment |
|
Neural Network |
Local processing, trained on 500 samples |
Date stamp character recognition |
|
MES Integration |
JSON data exchange protocol over OPC-UA |
Expiry date generation and verification |
|
Workflow Control |
Pass/fail signal to manufacturing line |
Automated process control |
Training and Validation Methodology
The neural network training approach focused on real-world operational conditions:
- 500 syringe samples representing 125 days of operational variation
- Multiple lighting conditions to simulate production environment
- Various stamping pressures and ink consistency levels
- Different operators and shift conditions included
Implementation Process
POC Execution Framework
The project followed a disciplined approach designed to minimize risk while maximizing learning:
POC Investment Breakdown
-
Total Budget
-
$25,000 allocated across 3 months
-
Hardware
-
Specialized camera and lightbox equipment
-
Software
-
Neural network development and training platform
-
Engineering Time
-
Integration development and testing resources
-
Risk Mitigation
-
Limited scope prevented larger pilot investment
Success Criteria Definition
Clear metrics were established before POC initiation:
- Zero false positives - no good parts incorrectly rejected
- Minimal false negatives - acceptable miss rate for defective stamps
- Integration feasibility - seamless MES connectivity
- Speed requirements - match or exceed human verification time
Results and Critical Analysis
The Technical Reality
Laboratory testing revealed both the promise and limitations of the AI vision approach:
|
Metric |
Target |
Achieved |
Assessment |
|---|---|---|---|
|
False Positives |
0% |
0% |
✓ Target Met |
|
False Negatives |
<5% |
Too High |
✗ Failed Criteria |
|
Integration |
Seamless |
Functional |
✓ Technical Success |
|
Processing Speed |
<30 seconds |
15 seconds |
✓ Performance Exceeded |
Root Cause Analysis
The excessive false negative rate stemmed from fundamental challenges in the production environment:
Critical Failure Points Identified
- Lighting variations - Production conditions differed from laboratory setup
- Magnification challenges - Similar digits difficult to distinguish consistently
- Stamp quality variations - Ink consistency and pressure differences
- Training data limitations - 500 samples insufficient for production variation
Despite technical feasibility, the system couldn't achieve the reliability required for FDA-regulated manufacturing where quality escapes carry significant risk.
The Strategic Pivot
Alternative Solution Implementation
Rather than proceeding to a costly pilot phase, the team implemented a human-centered approach:
"Sometimes the best technology solution is recognizing when people remain the optimal answer. The Andon cord approach preserved human expertise while addressing our speed concerns."
Andon Cord System Benefits
- Faster supervisor response - reduced changeover verification time
- Preserved human judgment - maintained quality expertise
- Cost-effective implementation - minimal technology investment
- Better operational fit - aligned with existing workflows
Financial Impact Comparison
|
Approach |
Implementation Cost |
Risk Level |
Time to Value |
Outcome |
|---|---|---|---|---|
|
AI Vision Pilot |
$150,000+ estimated |
High |
9-12 months |
Avoided |
|
AI Vision POC |
$25,000 |
Low |
3 months |
Learning achieved |
|
Andon System |
<$5,000 |
Minimal |
4 weeks |
Implemented |
Lessons Learned and Strategic Framework
Craig's Innovation Methodology Validated
This case study perfectly demonstrates the value of disciplined experimentation in manufacturing environments:
"Think Big, Start Small, Fail Fast, Scale What Works"
- Think Big - Envisioned fully automated quality verification eliminating dual signatures
- Start Small - Limited investment to $25,000 POC over 3 months
- Fail Fast - Recognized technical limitations within POC timeframe
- Scale What Works - Implemented human-centered Andon solution instead
Risk Management Excellence
The POC structure prevented a common industry failure pattern. Research shows that 88% of AI pilots fail to reach production, often after significant investment. This case avoided that trap through:
- Clear success criteria established before technical work began
- Limited financial exposure through POC-first approach
- Stakeholder expectation management - failure positioned as learning
- Alternative solution readiness - Andon approach developed in parallel
Organizational Culture Impact
"The failure was perceived as part of the cost of continuous improvement. This cultural acceptance of intelligent failure is crucial for innovation in regulated industries."
Industry Implications for Medical Device Manufacturers
When AI Isn't the Answer
This case provides valuable guidance for medical device manufacturers evaluating computer vision solutions:
Environmental Complexity Factors
- FDA-regulated environments require extremely low error rates
- Production lighting variations challenge laboratory-trained models
- Human expertise value often underestimated in automation planning
- Integration complexity can exceed technical feasibility challenges
Smart Technology Evaluation Criteria
|
Factor |
High AI Potential |
Human-Centered Approach |
|---|---|---|
|
Task Complexity |
Repetitive, rule-based |
Judgment-intensive, variable |
|
Error Tolerance |
Some false positives acceptable |
Zero tolerance for escapes |
|
Environment |
Controlled conditions |
Variable production settings |
|
Training Data |
Abundant, representative |
Limited, high variation |
Strategic Technology Planning
This POC informed the company's broader digital transformation strategy:
- AI evaluation capability - internal expertise developed for future assessments
- Risk management framework - POC-first approach standardized
- Vendor relationship building - technology partnerships established
- Innovation culture strengthening - intelligent failure celebrated
Conclusion: The Value of Intelligent Failure
While the AI vision system failed to meet production requirements, the POC delivered exceptional value through risk mitigation and strategic learning. The $25,000 investment prevented a potential $150,000+ pilot failure while accelerating implementation of an effective human-centered solution.
Final Project Outcomes
-
Financial Impact
-
$125,000+ avoided through prevented pilot investment
-
Timeline Benefit
-
6-9 months saved by avoiding failed pilot phase
-
Operational Improvement
-
Andon system reduced changeover verification time
-
Strategic Learning
-
Framework established for future AI evaluations
-
Cultural Development
-
Innovation mindset strengthened through intelligent failure
For medical device manufacturers facing similar automation decisions, this case demonstrates that smart experimentation methodology often delivers more value than successful technology implementation. The key is building organizational capability to fail fast, learn quickly, and scale what actually works - whether that's AI or enhanced human processes.
"POCs and pilots are all part of the experimentation lifecycle. The goal isn't always to prove the technology works - sometimes the greatest value comes from proving it doesn't, before you invest too much to change course."