IIoT
Industrial Internet of Things
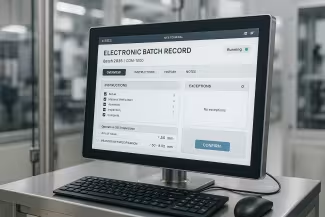
Case Study: MES Digitizes 1,800 Pages of Daily FDA Paperwork
Leading medical device manufacturer eliminated 1,800 pages of daily paperwork by implementing Critical Manufacturing MES across extreme vertical integration from weigh/dispense through tertiary packaging. The 24-month implementation achieved 66% faster changeovers, full CFR 21 Part 11 compliance, and eliminated mislabeling incidents. Key success factors included dedicated MES modeling teams, agile development methodology, and comprehensive integration with Oracle EBS, Agile PLM, and OPC-UA devices. The multi-million dollar investment delivered measurable ROI through operational efficiency, quality improvements, and FDA audit readiness.